Heisenberg uncertainty principle
It states that “It is impossible to determine the position and momentum of microscopic particles like an electron simultaneously and accurately”.
Mathematically,
\Delta x \cdot \Delta y \geq \frac{h}{4\pi}\\ \Delta x = uncertainty\ in\ position\\ \Delta y = uncertainty\ in\ momentumUncertainty means
i. If we measure the position more accurately, then the uncertainty in momentum occurs.
ii. If we measure the momentum more accurately, then the uncertainty in position occurs.
Concept of orbital
The space around the nucleus where the probability of finding the electron is maximum is called orbital.
Differences between orbit and orbital
| Orbit | Orbital |
| It is the well defined circular path where the electron revolves around the nucleus. | It is the space around the nucleus where the probability of finding the electron is maximum. |
| Orbits are circular in shape. | Orbitals have a different shape. |
| Orbit uses a two-dimensional concept. | Orbitals use three-dimensional concept. |
| Orbits don’t have directional characteristics. | Orbitals have directional characteristics except for s-orbitals. |
| One orbit can accommodate a maximum of 2n2 number of electron where n is the principal quantum number. | One orbital can accommodate a maximum of two electrons. |
| Orbit concept can’t explain the geometry of a molecule. | The orbital concept explains the geometry of a molecule. |
| It is developed by Bohr’s atomic model. | It is developed by the Heisenberg uncertainty principle. |
Shape of s and p orbital
s-orbitals are spherical in shape and p-orbitals are dumb-bell shape.
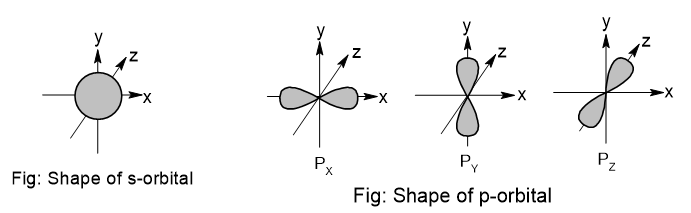
Quantum numbers
The quantum mechanical parameters which tell us about the location and motion of an electron in an atom are called quantum numbers. It gives complete information of a particular electron relating to its energy level, shape and orientation of orbital with its spin. The quantum number are as follows:
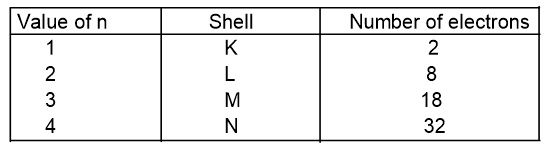
1. Principal quantum number (n)
It represents the number of shells or orbit or energy level in an atom. One orbit can accommodate a maximum of 2n2 number of electron where n is principal quantum number (given by Bohr Bury rule).
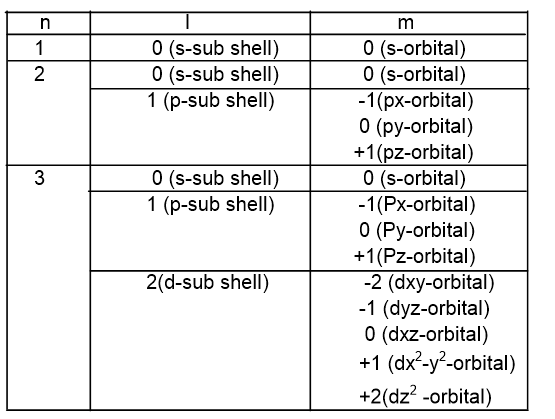
2. Azimuthal quantum number (l)
It describes the sub-shell or sub energy level. Its value lies from 0 to (n-1) for the value of n. It is also called a subsidiary quantum number.
3. Magnetic quantum number (m)
It gives information about the number of orbitals in a sub-shell. For the value of l, there are (2l+1) number of orbitals with the value of m from -l to +l.
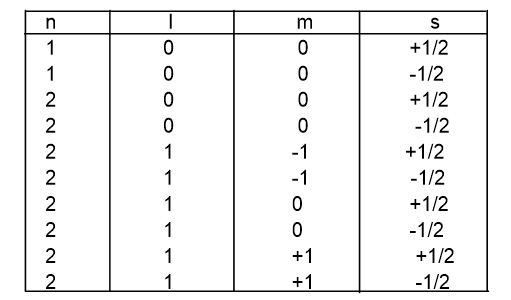
4. Spin quantum number
It denotes the spin of an electron on its own axis. For clockwise spin, +1/2 and for an anti-clockwise spin, -1/2 is assigned.
Arrangement of electron in atomic orbitals
1. Pauli’s exclusion principle
It states that “No two electrons in an atom can have identical set of four quantum numbers”.
2. Hund’s rule for maximum multiplicity
It states that “In an orbital of the same sub-shell, electrons are filled singly first before pairing starts”. Illustration: Let us take an example of the filling of electrons in a nitrogen atom. The electronic configuration of nitrogen is 1s 2s 2p. There are the following possibilities in filling the electrons in 2p.

3. Aufbau principle
It states that “The orbitals are filled up with electrons in the increasing order of their energy”.
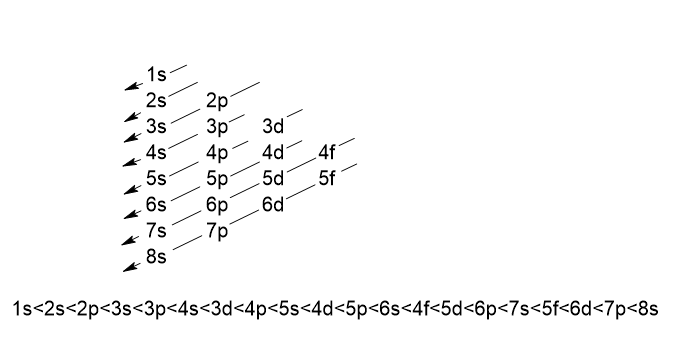
The above sequence can also be satisfied by the Madelung rule which is also called the (n+l) rule.
According to the rule:
- The orbitals with lower (n+I) value has lower energy than the orbitals of higher (n+l) value.
For 4s orbital, n = 4, l = 0, n+l=4
For 3d orbital, n = 3, l = 2, n+l=5 - When two orbitals have the same (n+l) value, the orbitals with a lower value of n has lower energy.
For 3d orbital, n = 3, l = 2, n+l=5
For 4p orbital, n = 4, l = 1, n+l=5
3d orbitals have less value of n. so it is filled first.
Limitations of Aufbau principle
Exactly half filled and full filled orbitals have greater stability than other due to following reasons:
- Symmetry: Half filled and full filled orbitals are more symmetrical than any other and symmetry lead to stability.
- Exchange energy: The electrons present in different orbitals of the same subshell can exchange their position. Each such exchange leads to a decrease in energy known as exchange energy. Greater the number of exchanges, the greater the exchange energy and the greater the stability.
Electronic configuration of elements in ground state

Electronic configuration of some ions
Fe++ = 1s2 2s2 2p6 3s2 3p6 4s0 3d6
Fe+++ = 1s2 2s2 2p6 3s2 3p6 4s0 3d5
Q. Write all quantum numbers for 3d and 2p .
| For 3d : n = 3 l = 2 m = -2, -1, 0, +1, +2 s = +1/2, -1/2 | For 2p : n = 2 l = 1 m= -1, 0, +1 (any one ) s = +1/2 or -1/2 |
Some Important Questions
- What are the observations of Rutherford’s atomic model?
- What are the postulates of Bohr’s atomic model?
- Draw a well-labelled diagram showing different spectra of the hydrogen atom.
- What is dual nature? State Heisenberg uncertainty principle.
- State Hund’s rule, aufbau rule and Pauli’s rule.
- Write all quantum numbers of 3s1, 2p4 and 3d3.
- Write the electronic configuration of iron in Fe(OH)2 and Fe(OH)3.
- What are degenerate orbitals? What is the quantization of angular momentum means?


