Variation of ionization energy in a period
On moving from left to right in a period, nuclear charge increases and the size of the atom decreases due to which electrons are more strongly attracted by the nucleus and more energy is required to remove the electron hence ionization energy increases.
Variation of ionization energy in a group
On moving from top to bottom in a group, the number of shells increases. So atomic size increases. Hence electrons are loosely attracted by the nucleus and consequently, ionization energy decreases.
- Alkali metal has the lowest ionization energy in the period. This is because of the largest atomic size and lowest nuclear charge.
- Noble gas has the highest ionization energy in the period. This is because of the highly stable electronic configuration. Helium is the element having the highest ionization energy in the periodic table.
- The ionization energy of beryllium (1s22s2) is more than that of boron (1s22s22p1). This is because s electrons are more strongly attracted by the nucleus than the corresponding p electrons in a given shell.
- The ionization energy of nitrogen(1s22s22p3) is more than that of oxygen (1s22s22p4). This is because nitrogen possesses half-filled orbitals which are more stable than the configuration of oxygen.
Electron affinity
The amount of energy released when an electron is added to an isolated gaseous atom in its ground state is called the electron affinity of that element.
Cl(g) + e– → Cl– + 348KJmol-1(EA)
Factors affecting electron affinity
1. Atomic size: As the size of atom increases, the force of attraction between the nucleus and electron decreases. Hence electron affinity decreases.
2. Nuclear charge: As the nuclear charge increases, the force of attraction between the nucleus and incoming electron increases and the electron affinity increases.
3. Electronic configuration: Elements having exactly half filled or completely filled orbitals are very stable. As a result they have little or no tendancy to add an electron and electron affinities are low or almost zero in this case.
Variation of electron affinity in a period
On moving from left to right in a period, nuclear charge increases and the size of the atom decreases due to which tendency of an atom to attract incoming electron increases. So electron affinity increases.
Variation of electron affinity in a group
On moving from top to bottom in a group, the number of shell increases and the size of the atom is increased due to which the tendency of an atom to attract incoming electron decreases so electron affinity decreases.
- Halogen has the highest electron affinity in the period. This is because of the smallest atomic size and high nuclear charge.
- Noble gas has zero electron affinity. This is because of the highly stable electronic configuration.
- The electron affinity of the IIA group is almost zero.
- Electron affinity of Nitrogen and phosphorus are very low due to the stability of half-filled orbitals.
- The electron affinity of fluorine is less than that of chlorine. Electron density in fluorine is high due to its very small size. Hence the adding electron experience more repulsion of the electron cloud of fluorine and some extra energy has to be supplied for adding electron. Therefore net energy released is less. Chlorine being relatively large in size and electron density is not so high and therefore less repulsion takes place.
Electronegativity
The electronegativity of an element is the tendency of an atom in a molecule to attract the pair of electrons towards itself. It is not the property of an isolated atom.
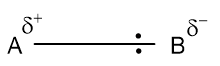
Here, B attracts the shared pair more than A does. In this case, B is said to be more electronegative than A.
Variation of electronegativity in a period
On moving from left to right in a period, nuclear charge increases and the size of the atom decreases due to which the tendency to attract the shared pair of electron increases. So electronegativity increases.
| Li | Be | B | C | N | O | F |
| 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 |
Variation of electronegativity in a group
On moving from top to bottom in a group, the size of the atom increases due to which the tendency of an atom to attract shared pair of electrons decreases. Hence electronegativity decreases.
F → 4
Cl → 3
Br → 2.8
I → 2.5
| IE | EA | EN | |
| Period | Increases | Increases | Increases |
| Group | Decreases | Decreases | Decreases |
Some Important Questions
- State modern periodic law. Write the demerits of the modern periodic table.
- Define periodicity. Why the atomic radius of He is greater than H?
- Compare the size with explanation:
a. Li and C
b. F and Cl
c. K+ and Ca++
d. F and F–
e. Fe++ and Fe+++
f. S– and Cl–
g. Na+ and Mg++ - What is ionization energy? Compare the ionization energies of elements of period 2.
- What is electron affinity? Compare the electron affinity of F and Cl.
- What are transition elements? Write the name of transition elements that lies in period 4.


