10. An organic compound P which reduces Tollen’s reagent. On oxidation with KMnO4, it formed a compound Q that has the same number of carbon atoms as P. Q reacts with Na2CO3 to give CO2. Q on reaction with ethanol in the presence of conc. H2SO4 formed an ester having molecular formula C4H8O2(R).

11. An organic compound A reacts with sodium metal to give hydrogen gas. The compound A, on treatment with alkaline iodine, forms a yellow crystalline substance, and on oxidation with acidified dichromate forms an aldehyde with molecular formula C2H4O.

12. An aliphatic compound A reacts with aq. NaOH to give B. B on oxidation with K2Cr2O7/H+ produces C. C undergoes Clemmensen reduction to give propane. The compound C responds positively to iodoform test.

13. An aliphatic compound A reacts with SOCl2 to give B. B, on dehydrohalogenation, yields C. C, on ozonolysis, gives a mixture of ethanal and methanal. A is alcohol that responds to iodoform test.

14. An alkene A undergoes ozonolysis to give an aldehyde and a ketone. The aldehyde gives a positive iodoform reaction, and the ketone undergoes Clemmensen reduction to yield propane.

15. Consider the following:

The compound E produces ethyne when heated with silver powder.

16. Convert:
i. Ethanoic acid into methanoic acid

ii. Methanoic acid to ethanoic acid

17. Consider the following:

The compound E is a primary alcohol, and it has a positive iodoform test.

18. Identify A, B, C and D from the given conversion:


19. Consider the given conversion:

The compound E can be obtained by heating oxalic acid in the presence of glycerol.

20. An organic compound A having molecular formula C3H7Cl on reaction with alc. KCN gives B. B on hydrolysis with dil. HCl gives compound C, and on reduction with H2/Ni, gives 1-amino butane.

21. An organic compound A (C3H6O) is resistant to oxidation but forms compound B (C3H8O) on reduction, which reacts with HBr to form the bromide C. C forms a Grignard’s reagent that reacts with A to give D (C6H14O).
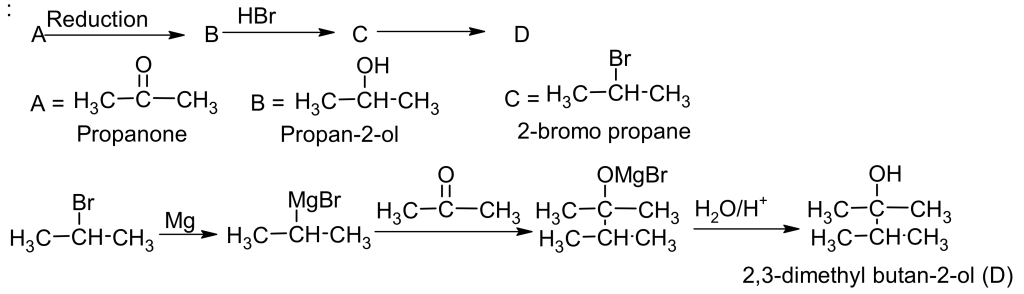
22. A compound A with molecular formula C5H12O on oxidation forms B (C5H10O). B gives the iodoform test but does not reduce the ammonical AgNO3 solution. B, on reduction with amalgamated zinc and HCl, gives compound C with molecular formula C5H12.

23. An organic compound A (C5H12O) reacts with sodium metal to give hydrogen gas. A on treatment with hot conc. H2SO4 gives B (C5H10), which on ozonolysis yields propanone and ethanal.

24. An organic compound A of molecular formula C2H6O reacts with sodium metal to form a compound B with the evolution of hydrogen gas. It gives yellow compound C when warmed with iodine and NaOH. When heated with conc. H2SO4 at 140 °C, A gives D (C4H10O), which on treatment with conc. HI at 100 °C gives E. D is also obtained when B is treated with E.

25. A liquid A (C6H10O2) upon acid hydrolysis gives a carboxylic acid B and an alcohol C. Oxidation of C with chromic acid gives B.

Go to the next page for the remaining important reactions


