Aniline
The organic compound which has an amino group attached to the phenyl group is called aniline. Its general formula is C6H5NH2.
General methods of preparation of aniline
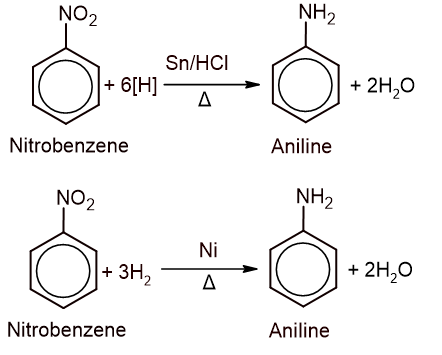
1. From nitrobenzene: Aniline is prepared either by the reduction of nitrobenzene with granulated tin and concentrated hydrochloric acid or catalytic hydrogenation.
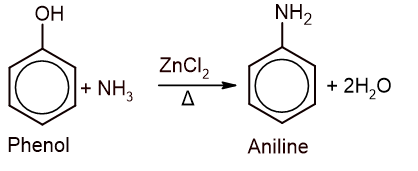
2. From phenol: When phenol is reacted with ammonia in presence of zinc chloride at 300°C and high pressure, aniline is formed.
Physical properties of aniline
- It is colourless liquid with unpleasent odour.
- It is insoluble in water.
- It’s boiling point is 184°C.
- It is steam volatile and poisonous.
Chemical properties
1. Basicity of aniline: Aniline is basic in nature due to the presence of lone pair of electrons on a nitrogen atom. However, it is less basic than ammonia and aliphatic amines because its lone pair of electrons present in the nitrogen atom takes part in resonance.
Aliphatic amine > Ammonia > Aromatic amine
Decreasing order of basic
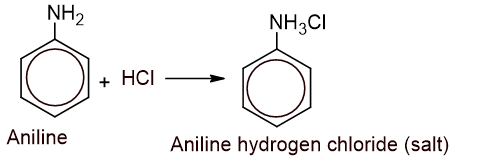
- Action with HCl
Effect of substitution in basic nature of aniline
- Presence of an electron-withdrawing group in pare position decreases the basic strength of aniline.
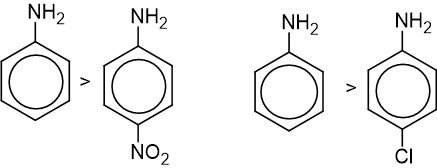
- Presence of electron releasing group in para position increases the basic nature of aniline.
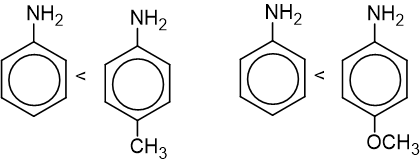
- Presence of any substituents in the ortho position decreases the basic nature of aniline.
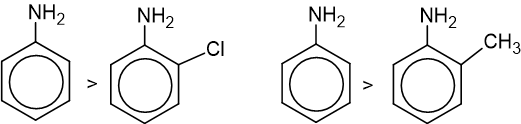
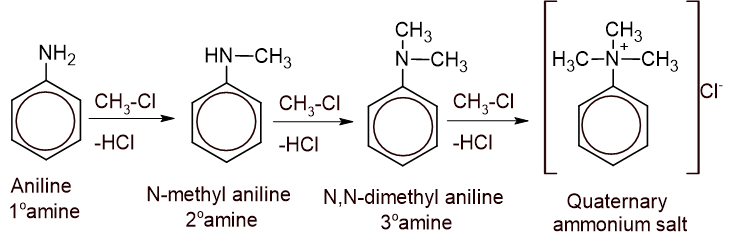
2. Alkylation: Aniline reacts with an alkyl halide gives a mixture of secondary, tertiary amine and quaternary ammonium salt.
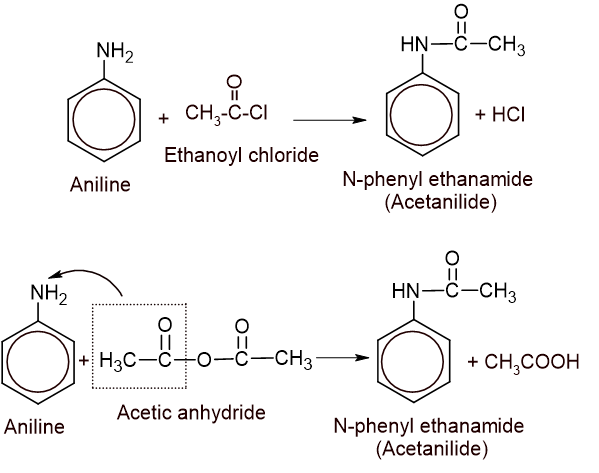
3. Acylation: Aniline reacts with acid chloride or anhydride to give N-phenyl ethanamide (acetanilide).
4. Carbylamine reaction
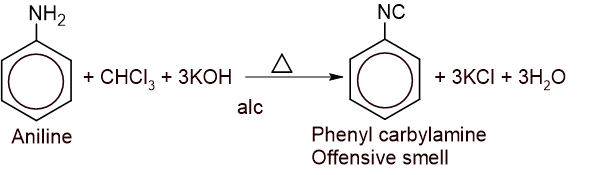
5. Diazotization reaction: Aniline is reacted with nitrous acid in cold conditions to give benzene diazonium chloride.

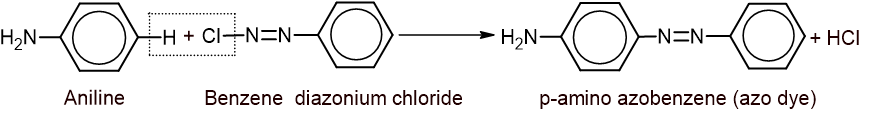
6. Coupling reaction: Aniline reacts with benzene diazonium chloride in slight acidic medium to give p-amino azobenzene which is also known as azo dye.
Electrophilic substitution reaction
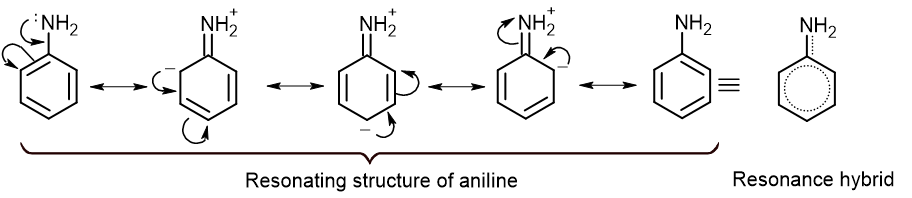
From the above resonating structure, it is clear that -NH2 group increases the electron density at ortho and para position. So, incoming electrophile prefers to attack at ortho and para position. Hence, -NH2 group is the ortho para directing group.
Since, -NH2 group increases the electron density in the ring, it is also called activating group.
1. Bromination: Aniline reacts with aqueous bromine to form a white precipitate of 2,4,6-tribromo aniline.
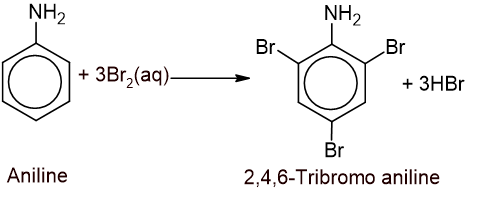
In this reaction, the -NH2 group highly activates the ring.
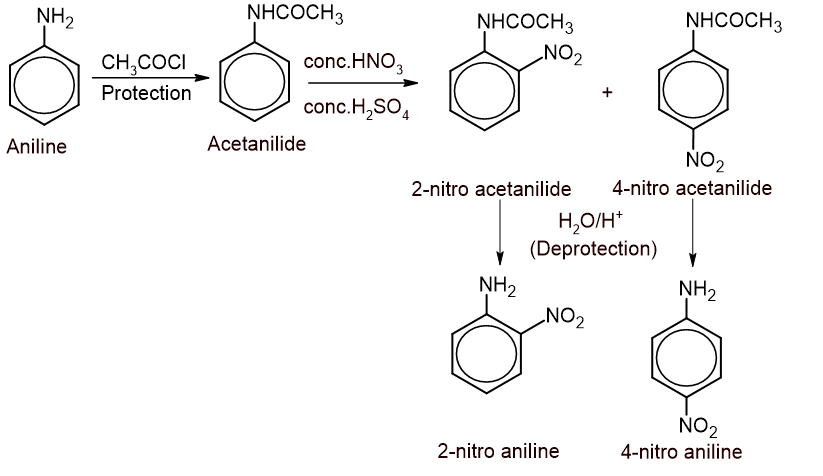
To get a monosubstituted product, a highly activating amino group is protected by acylation and then halogenation is carried out followed by hydrolysis to regenerate the amino group.

2. Nitration: Direct nitration of aniline cannot be carried out because conc. nitric acid being a strong oxidizing agent oxidizes aniline into the various oxidized products. So, the amino group is first protected by acylation before nitration followed by hydrolysis to regenerate the amino group.
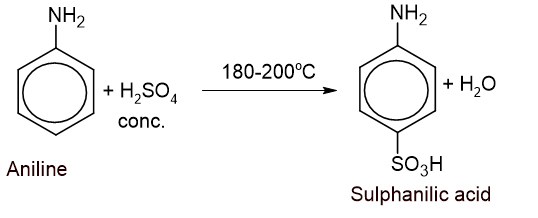
3. Sulphonation: Aniline is heated with conc. sulphuric acid at 180-200°C for 4-5 hours to give p-amino benzene sulphonic acid (sulphanilic acid).
Uses of aniline
- In the manufacture of dyes and drugs.
- For the preparation of acetanilide, sulphanilic acid, etc.
- For preparation of antioxidant in rubber industry.


